
#Bio rad western blot gel preparation verification#
Verification of Western Blotting Protein Transfer Using Stain-Free ImagingĪssessment of protein transfer using a stain-free enabled imaging system. This allows visualization, verification, and validation at all steps of electrophoresis and blotting, potentially saving time wasted on western blots with problems that would not otherwise have been detected until the later stages of blot processing and development. Images of the gel or membrane after transfer can easily be captured multiple times without staining and destaining steps. Stain-free imaging technology utilizes a proprietary trihalo compound to enhance natural protein fluorescence by covalently binding to tryptophan residues with a brief UV activation. The invention of stain-free imaging technology has made total protein normalization a much better alternative for quantitative western blotting. Unfortunately, the use of total protein stains can interfere with subsequent blot development and visualization steps, typically requiring tedious staining and destaining procedures, a major reason for the lack of widespread adoption of total protein normalization using these stains. Therefore, both low-abundance target protein levels, measured using sensitive immunodetection techniques, and high-abundance housekeeping protein levels, measured using less sensitive total protein staining techniques, can each be measured in their respective linear dynamic ranges. As a result, they exhibit good linearity in the common loading range of 10–50 μg of cell lysate. Total protein levels can be determined by staining the membrane with total protein stains, e.g., Coomassie, SYPRO Ruby, Flamingo, amido black, or Ponceau S.īecause total protein stains are less sensitive than antibody-based immunodetection, they are far less likely to result in an oversaturated signal. The use of total protein measurement for western blot loading controls (total protein normalization TPN) is a method devised to resolve the inherent difficulties with linearity in the immunodetection of both target and control proteins. Total Protein Normalization: The Better Alternative Common housekeeping proteins are upregulated in colorectal adenocarcinoma and hepatocellular carcinoma, making the total protein a better "housekeeper". Total protein normalization of HKPs yields consistent band intensities.

Immunodetection measurements of housekeeping protein levels show poor linearity and do not accurately indicate cell lysate loading levels. The expression level of housekeeping proteins can change due to:ĭifferences in five candidate housekeeping proteins and total protein staining between tumor and non-cancerous tissues in the validation sample set. Variation in Housekeeping Protein (HKP) Expression Levels Furthermore, HKP expression levels may not be constant but instead vary with different experimental treatments and other factors.

Thus, to detect the target protein of interest, large amounts of cell lysate may need to be loaded, resulting in overloading of HKPs, yielding oversaturated reference bands, out of their linear range. Unfortunately, housekeeping proteins are usually highly expressed, whereas target proteins are often expressed only in low abundance.
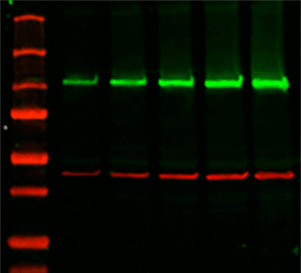
Reliable assessment of the changes in target protein expression levels requires the measurement of both the target protein and the loading control protein in their linear dynamic ranges for immunodetection. Typically, a "housekeeping protein" (HKP) such as β-actin, β-tubulin, or GAPDH is used as a loading control, with the assumption that the expression levels of these proteins remains constant. Proper western blot normalization is required to show that the changes in band intensities correlate to the biological changes in your samples.Ĭonventional loading controls rely on consistent levels of a reference protein in each sample. Western blot normalization allows you to faithfully compare changes in protein expression by establishing the baseline needed to correct against common errors such as inconsistent sample preparation, pipetting error, and uneven protein transfer.


 0 kommentar(er)
0 kommentar(er)
